Chirality
|
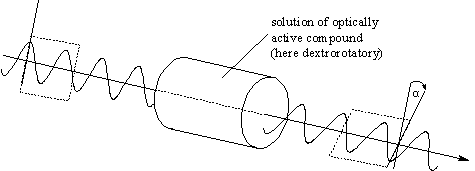
A 1:1 mixture of enantiomers is called racemic or a racemate. A chiral nonracemic compound is a sample of a chiral compound containing an excess of one enantiomer, either a small excess or an almost enantiopure sample. The simplest context in which enantiomers differ is the so-called optical activity. By this the phenomenon is understood that a chiral nonracemic compound in solution or as a pure liquid will influence the plane of linearly polarized light, i.e. rotates that plane by a certain angle ("optical rotation"). One pure enantiomer rotates clockwise by a certain angle, the other one counterclockwise by the same angle ("dextrorotatory", 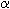 > 0;
"laevorotatory", > 0;
"laevorotatory", 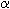 < 0, the observer is looking against the beam
of light), under well-defined conditions. < 0, the observer is looking against the beam
of light), under well-defined conditions.
For these reasons we have to differentiate chirality and optical activity. |