|
The composition of a mixture of enantiomers may be measured
and described as a numerical value. The particular notation
depends on the measurement procedure.
In early times optical rotation was the only property amenable to
measurement. The outcome of such a measurement was the specific
rotation of a compound sample
[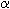 ] = 100 ] = 100 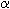 / (c·d) , / (c·d) ,
|
where 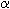 is the measured angle
of rotation (°), c is the concentration of the sample solution (g/100 ml
solution), and d is the length (dm) of the cuvette containing the sample
solution. The product c·d is proportional to the mass amount of solute which
interacts with the beam of polarized light, it appears in the denominater in
order that [ is the measured angle
of rotation (°), c is the concentration of the sample solution (g/100 ml
solution), and d is the length (dm) of the cuvette containing the sample
solution. The product c·d is proportional to the mass amount of solute which
interacts with the beam of polarized light, it appears in the denominater in
order that [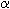 ] be proportional
to the rotatory power of a constant amount. Since the rotatory power is dependent
on the wavelength of light used and on the temperature, these parameters have to
be given, e.g. in the form [ ] be proportional
to the rotatory power of a constant amount. Since the rotatory power is dependent
on the wavelength of light used and on the temperature, these parameters have to
be given, e.g. in the form [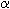 ]D20. D here is the wavelength of the sodium D line
(589 nm), 20 stands for 20°C. Further the rotatory power depends on solvent and
concentration, so that these have also to be given (even though a division by c
was already done, the reason for that are concentration-dependent association
phenomena). ]D20. D here is the wavelength of the sodium D line
(589 nm), 20 stands for 20°C. Further the rotatory power depends on solvent and
concentration, so that these have also to be given (even though a division by c
was already done, the reason for that are concentration-dependent association
phenomena).
Example: [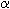 ]D20 = -13.5
(c=1.3; ethanol) for a particular sample of (R)-2-butanol.
The minus sign means laevorotatory, i.e. counterclockwise if the observer's
view is opposite to the incoming beam of light. ]D20 = -13.5
(c=1.3; ethanol) for a particular sample of (R)-2-butanol.
The minus sign means laevorotatory, i.e. counterclockwise if the observer's
view is opposite to the incoming beam of light.
| 



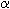 ] = 100
] = 100