Chirality: Fischer projection
|
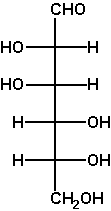
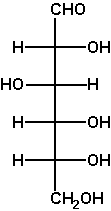
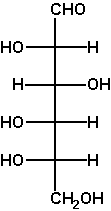
The Fischer projection enables us to simply read or represent enantiomer or diastereomer relations among complex acyclic molecules containing several asymmetric C atoms. This is its foremost advantage. For example, D-mannose differs from D-glucose in the configuration at C2 only (two diastereomers), while L-glucose is the enantiomer of D-glucose.
Verify these statements by performing the said operations on a Fischer projection (e.g. of D-glyceraldehyde OCH-CH(OH)-CH2OH) and translating the obtained projection into a 3D formula. |