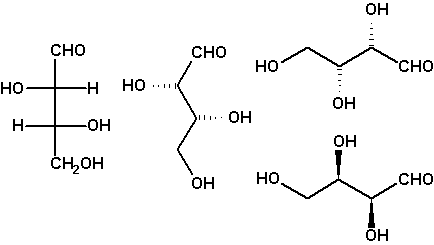
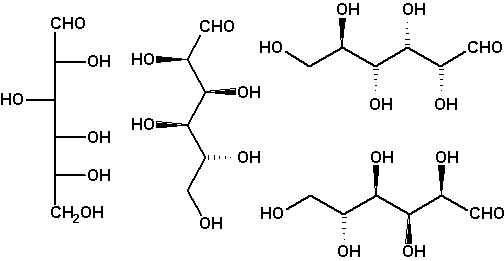
Chirality: Fischer projection
|
A much more realistic picture of an open-chain molecule than provided by a Fischer projection is a zigzag projection. It depicts the main chain in the all-antiperiplanar conformation (all C-C-C-C torsion angles 180°). In a zigzag projection, the main chain carbons are positioned in the paper plane, the two other substituents at each carbon pointing up and down. Usually non-H substituents only are drawn. Two vicinal substituents that have an erythro relation in the Fischer projection (are on the same side of the vertical backbone) are anti in the zigzag projection (one up, one down), and two vicinal substituents threo in the Fischer projection (on different sides of the backbone) are syn in the zigzag projection (both up or both down). Since a zigzag projection is a perspective picture not loaden by any conventions, it may be rotated by any angle.
|