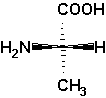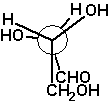|
The Fischer projection is a schematic representation
of a chiral molecule that for some purposes obviates perspective
drawings.
The conventions are as follows:
-
In a Fischer projection the compound's C chain is written
vertically [usually with the most oxidized carbon atom on top
(e.g. -COOH, if present; for an aldose the aldehyde group -CHO)].
-
The remaining substituents at each carbon are written
with horizontal bonds.
-
At each asymmetric carbon atom the vertical bonds are to be
imagined behind the paper plane, the horizontal ones in front
of the paper plane.
-
The internal carbon atoms often are not written.
-
H atoms as substituents often are not written.
-
A hetero atom bonded to the lowermost asymmetric C atom
attributes to the compound a D- or
an L-label (from Latin dexter,
right, and laevus, left).
Examples:
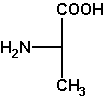
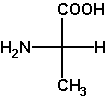
L-alanine
|
=
|
|
=
|
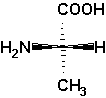
|
=
|
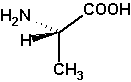
(S)-alanine
|
| |
|
|
|
|
|
|
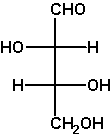
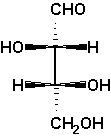
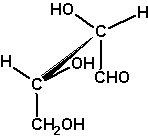
D-threose
|
=
|
|
=
|
|
=
|
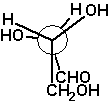
|
The example D-threose nicely demonstrates a
characteristic of the Fischer projection: It describes a molecule
in its extremely unrealistic all-eclipsed conformation of the main
chain, see the Newman projection at the right and the
sawhorse projection left to it.
|