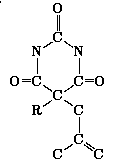
These are a group of compounds with hypnotic effects [30]. Here we will consider structures of the form:

In [12] a correlation was established between the minimum mol of drug (c) per kg of the test animal producing hypnosis and the SIC. On this data, taken from [31], regression analysis yields the following equation:
with correlation coefficient 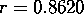 .
.
As residuals R alkanyl and some alkenyl groups were used. Therefore it is justified to extrapolate this correlation to compounds other than those mentioned there. We tried this with the full variety of alkanyls and alkenyls as substituents and found two interesting results:
 ,
the
,
the  calculated by eq. 3 is always minimal
for the n-alkanyl (see table 1). Any branched substituent
leads to a higher value. So the cases studied in [12]
were indeed the optimal ones.
calculated by eq. 3 is always minimal
for the n-alkanyl (see table 1). Any branched substituent
leads to a higher value. So the cases studied in [12]
were indeed the optimal ones.
This way, structure generation helps to reinforce conjectures and to show the scientist a direction for further investigations.
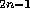 ,
we found a number of compounds the calculated
,
we found a number of compounds the calculated  of which is significantly larger than that of the structures
studied in [12] (see table 2).
Some of them are printed in the figures: One optimal isomer for
substituent C
of which is significantly larger than that of the structures
studied in [12] (see table 2).
Some of them are printed in the figures: One optimal isomer for
substituent C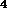 H
H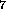 (fig. 1), three isomers for C
(fig. 1), three isomers for C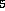 H
H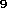 in fig. 2 and also three for C
in fig. 2 and also three for C H
H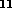 (fig. 3).
(fig. 3).
In this case, the extrapolation shows that the optimal structures can often be those that don't come into one's mind immediately. Although the statistical analysis that lead to eqn. 3 is only on a small data basis and should be refined and apart from other important aspects like how easy it is to synthesize, the structure generator allows to cover the whole variety of isomers and avoids overlooking important and especially active compounds.
wieland@btm2d1.mat.uni-bayreuth.de