Chirality
|
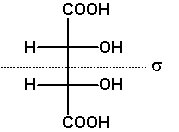
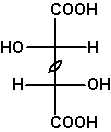
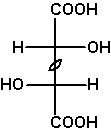
If the two constitutionally identical asymmetric carbons are of the same configuration, the compound is chiral, one enantiomer being RR, the other SS. These compounds and the meso compound are diastereomers.
The Fischer projection depicts the unrealistic eclipsed syn-periplanar conformation. It allows us to simply read the symmetry of this conformation, meso-tartaric acid Cs, (S,S)-tartaric acid and (R,R)-tartaric acid C2.
As an exercise, determine the symmetries of the other conformations of meso- and (R,R)-tartaric acids shown above. |