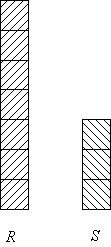
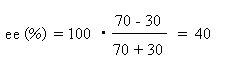Chirality: Optical purity vs ee
|
Since about 1975 it is possible to separate enantiomers chromatographically and to determine their amounts quantitatively, using so-called chiral columns in liquid or gas chromatography. Alternatively in certain NMR experiments both enantiomers can be observed and quantified side-by-side. By these methods the enantiomer ratio is immediately obtained. However, the ratio is not normally given, rather the quantity enantiomeric excess, ee, defined as follows:
This format of result has advantages:
Example: [R] : [S] = 70 : 30
|