Chirality: Thalidomide
|
Meanwhile it is known (by trials on healthy male volunteers) that the enantiomers of thalidomide racemize in the human body under physiological conditions within a few hours. This may be the clue to the contradictive findings mentioned above, but more importantly it renders obsolete the question for differences in teratogenicity between thalidomide enantiomers.
The easy racemization is understandable from the molecular structure:
The compound is a derivative of an
The enolate is achiral, its
|




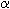 -amino acid, it contains an asymmetric carbon atom,
one substituent of which is a hydrogen atom. The asymmetric carbon is
located
-amino acid, it contains an asymmetric carbon atom,
one substituent of which is a hydrogen atom. The asymmetric carbon is
located