Chirality: Thalidomide
|
The sedative drug thalidomide was commercialized in the late
1950s / early 1960s, its active principle was racemic N-phthaloylglutaminimide
(
When incorporated by women in early pregnancy the drug caused severe malformations of the fetuses (teratogenicity). Today it is generally accepted that as a rule enantiomers differ in their properties. It seems therefore plausible that the sedative and the teratogenic properties may be due to a single enantiomer of thalidomide each, and these may even be different enantiomers. In many texts this possibility is claimed as a fact, though the final answer to this question is not known. There are reports on animal experiments both finding and not finding differences in teratogenicity between the enantiomers. |




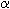 -phthalimidoglutarimide).
-phthalimidoglutarimide).